Position Statement & Case Definition Crosswalk
In 2019 the first CSTE position statement providing a Neonatal Abstinence Syndrome Standardized Case Definition was adopted as 19-MCH-01. After selected jurisdictions applied this case definition, it was determined that updates were needed to allow for clarity in interpretation, consistency in reporting across jurisdictions, and to address concerns identified during implementation. In 2023 an update to the 2019 position statement was adopted, 23-MCH-01.
Classification Updates Within The Position Statement
Classification Types
The sub-classifications of “Types” of probable and suspect cases were removed for simplicity.
Tier 2 Surveillance
For Tier 2 surveillance, the suspect category of case classification was removed.
Laboratory Results
Positive neonatal laboratory results for non-opioid, benzodiazepine, or barbiturate (non-OBB) substances are now included as supportive laboratory evidence.
Clinical Signs
The number of clinical signs of NAS needed to meet the criteria for clinical compatibility was lowered from three or more signs to two or more signs.
Maternal Laboratory Timeline
The timeline for positive maternal laboratory results or maternal history of use was updated from “within four weeks prior to delivery” to “within the current pregnancy” for history of use and “within four weeks prior to delivery through one day post-delivery” for laboratory testing.
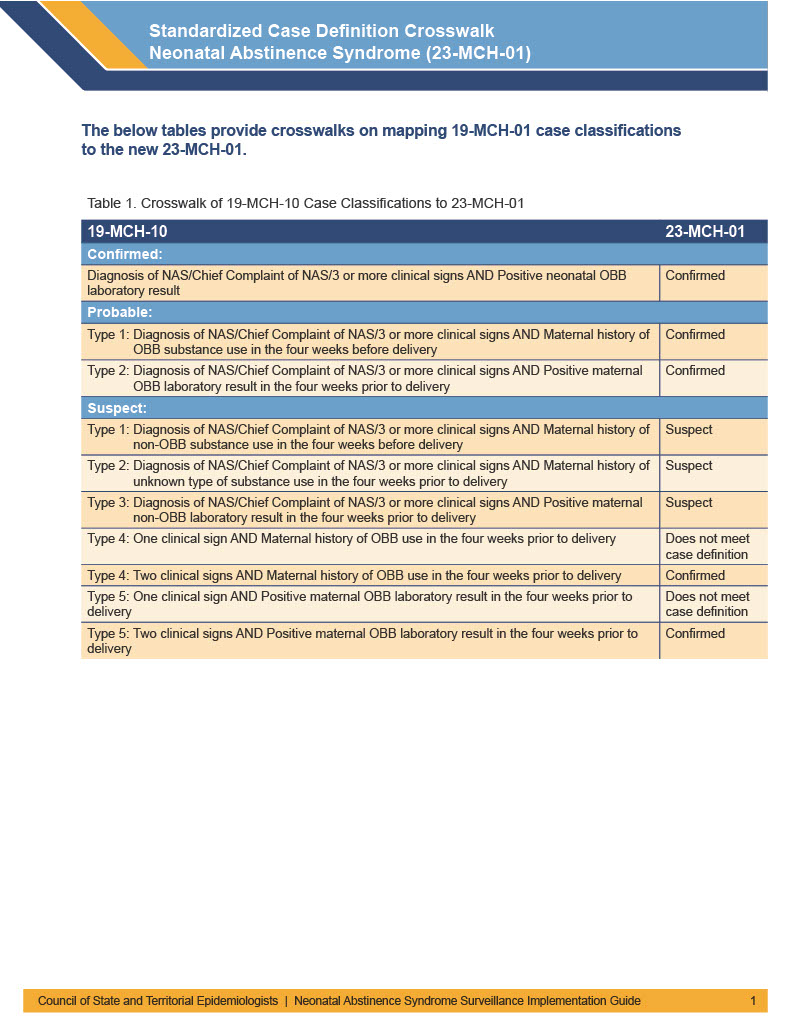
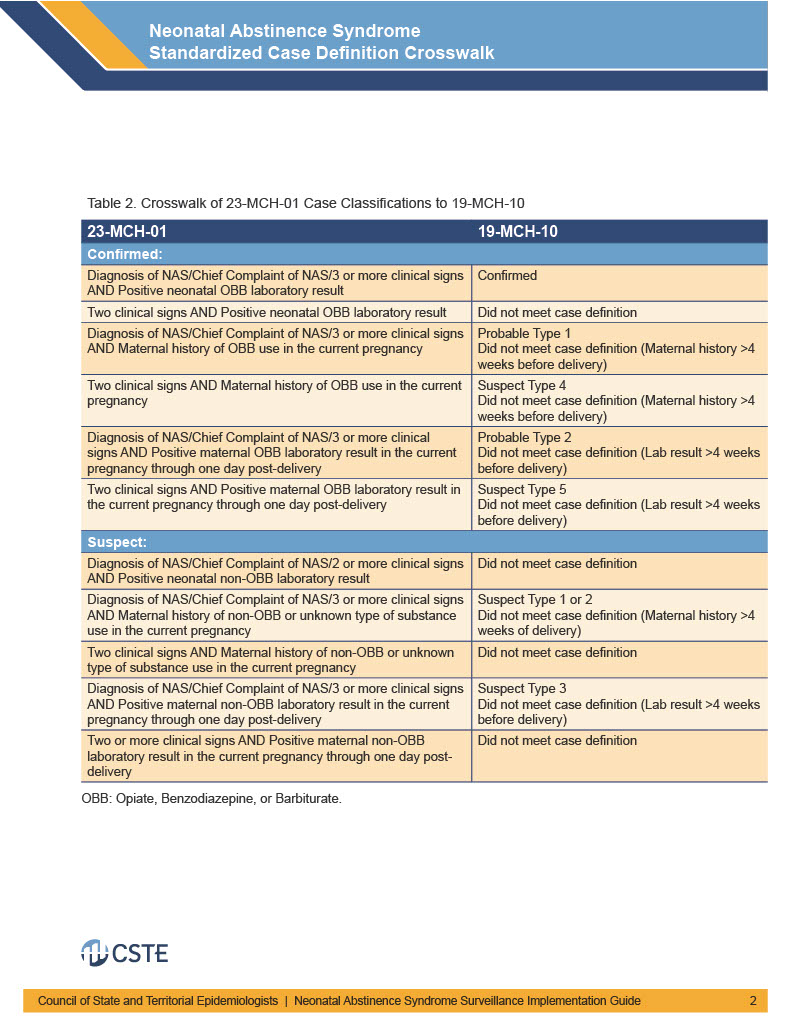
The below tables provide crosswalks on mapping 19-MCH-01 case classifications to the new definition, as well as explain within the new definition how the cases were previously classified.
Next, Annotated Case Classification
